The global virtual clinical trials market size is expected to reach USD 12.9 billion by 2030, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 5.7% from 2022 to 2030. The COVID-19 pandemic has significantly impacted the market. The growing need for patient diversity clubbed with enhanced data collection in clinical trials is boosting the market growth.
There has been a rise in the R&D of new drugs and vaccines that has increased the volume and complexity of clinical trials. Virtual clinical trials eliminate challenges posed by traditional clinical trials for example delays in patient recruitment and time-consuming procedures. Also, studies have revealed that around 75.0% of people favored a mobile trial over traditional ones and 80.0% of patients are more likely to participate in a clinical trial that uses mobile technology.
The market is recovering at a significant pace after the pandemic as it offers various benefits to patients as well as sponsors. During the pandemic, traveling was potentially dangerous. It can be expensive, even incurring lost wages or requiring that childcare/eldercare be hired. These drawbacks limit initial interest and provide a simple solution for virtual clinical trials. Virtual clinical trials offer various benefits such as effective data collection, analysis, and monitoring large amounts of data in real-time.
Virtual trials make use of monitoring devices, software apps, online social engagement platforms to conduct every step of the clinical trial process including patient recruitment, counseling, measuring clinical endpoints, informed consent, and adverse reactions. Telehealth, home care, and remote patient monitoring has been gaining momentum as a healthcare offering, and the COVID-19 is adding more horsepower to this initiative.
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/virtual-clinical-trials-market
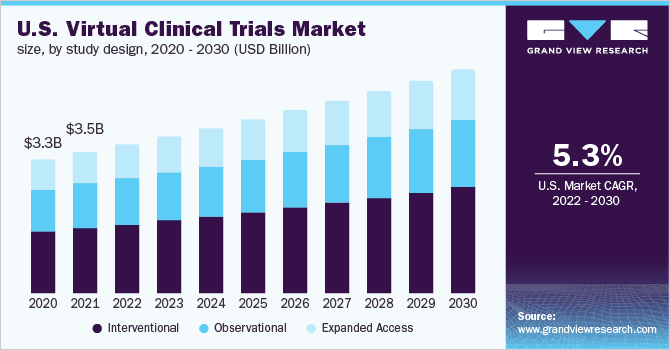
Virtual Clinical Trials Market Report Trends and Growth
- The oncology segment held 25.2% of the revenue share in 2021. The increasing adoption of virtual trials especially in oncology clinical research for the diverse populations is responsible for the growth of the segment
- The interventional design segment accounted for the largest revenue share of 46.7% in 2021
- North America held 49.2% of the revenue share in 2021. Favorable government initiatives and the presence of large numbers of players in the U.S. offerings advanced services are responsible for market growth
- In Asia Pacific, the market is expected to witness the fastest CAGR of 6.8% over the forecast period owing to the increasing patient pool and cost-efficient services
Key Companies and Market Share Insights
The market for global virtual clinical trials is highly competitive. Significant factors affecting competitive nature are the quick adoption of advanced technology for improved healthcare. Besides, players are also acquiring, collaborating, partnering with other firms to gain the market share. For instance, in May 2020, Covance announced expanding its technology ecosystem to accelerate decentralized clinical trials adoption. The company is doing so through an alliance with Medable, a prominent software provider for digital clinical trials. Some of the prominent players in the virtual clinical trials market include: ICON, Parexel International Corporation, IQVIA, Covance, PRA Health Sciences, LEO Innovation Lab, Medidata, Oracle, CRF Health, Clinical Ink, Medable, Signant Health, Clinical Ink, Halo Health Systems, Croprime
Request Free sample Report: https://www.grandviewresearch.com/industry-analysis/virtual-clinical-trials-market