The integration of genomic data into Real-World Evidence (RWE) applications Market is revolutionizing healthcare by enabling personalized medicine, enhancing drug development, and informing clinical decision-making. This convergence has led to a burgeoning market focused on leveraging genomic data for RWE purposes.
Market Overview
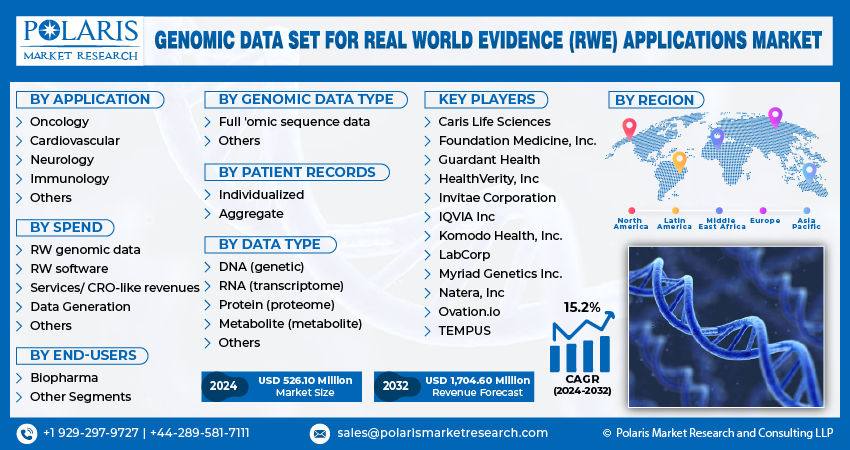
The global market for genomic data sets in RWE applications is experiencing significant growth. Projections indicate that the market size will reach approximately USD 1.7 billion by 2032, with a compound annual growth rate (CAGR) of 15.2% from 2024 to 2032.
Download Free Sample PDF Copy of the Report:
Some of the major players operating in the global Genomic Data Set for Real World Evidence (RWE) Applications Market include:
- Caris Life Sciences
- Foundation Medicine, Inc.
- Guardant Health
- HealthVerity, Inc
- Invitae Corporation
- IQVIA Inc
- Komodo Health, Inc.
- LabCorp
- Myriad Genetics Inc.
- Natera, Inc
- Ovation.io
- TEMPUS
Key Market Segments
Genomic Data Set for Real World Evidence (RWE) Applications Market, Application Outlook (Revenue, USD Million, 2019-2032)
- Oncology
- Cardiovascular
- Neurology
- Immunology
- Others
Genomic Data Set for Real World Evidence (RWE) Applications Market, Spend Outlook (Revenue, USD Million, 2019-2032)
- RW genomic data
- RW software
- Services/ CRO-like revenues
- Data Generation
- Others
Genomic Data Set for Real World Evidence (RWE) Applications Market, Data typers Type Outlook (Revenue, USD Million, 2019-2032)
- Biopharma
- Other Segments
Genomic Data Set for Real World Evidence (RWE) Applications Market, Genomic Data Type Outlook (Revenue, USD Million, 2019-2032)
- Full ‘omic sequence data
- Others
Genomic Data Set for Real World Evidence (RWE) Applications Market, Patient Records Type Outlook (Revenue, USD Million, 2019-2032)
- Individualized
- Aggregate
Genomic Data Set for Real World Evidence (RWE) Applications Market, Data type Outlook (Revenue, USD Million, 2019-2032)
- DNA (genetic)
- RNA (transcriptome)
- Protein (proteome)
- Metabolite (metabolite)
- Others
Market Growth Drivers
- Advancements in Genomic Technologies: Innovations in sequencing technologies have reduced costs and increased accessibility, facilitating the integration of genomic data into RWE.
- Emphasis on Personalized Medicine: The shift towards treatments tailored to individual genetic profiles has heightened the demand for comprehensive genomic data.
- Regulatory Support: Agencies like the U.S. Food and Drug Administration (FDA) are increasingly recognizing RWE in regulatory decision-making, encouraging the use of genomic data.
- Collaborative Initiatives: Partnerships between industry stakeholders and research institutions are promoting data sharing and the development of robust genomic databases.
Challenges
- Data Privacy and Security: The sensitive nature of genomic information raises concerns about privacy breaches and ethical considerations.
- Standardization Issues: The lack of uniform data formats and analytical methods can hinder the effective integration of genomic data into RWE.
Future Outlook
The market for genomic data sets in RWE applications is poised for substantial growth, driven by technological advancements and the increasing adoption of precision medicine. Ongoing efforts to address data privacy concerns and standardization challenges will be crucial in realizing the full potential of genomic data in real-world settings.
In conclusion, the integration of genomic data into RWE applications represents a transformative development in healthcare, offering the promise of more personalized and effective treatments. As the market continues to evolve, stakeholders must navigate challenges related to data privacy and standardization to fully harness the benefits of this integration.
Recent Developments in Genomic Data Sets for Real-World Evidence (RWE) Applications
April 2023: Natera, Inc. presented new data on its Signatera™ molecular residual disease (MRD) test at the American Association for Cancer Research (AACR) Annual Meeting. The findings, derived from five studies across various cancers—including esophago-gastric adenocarcinoma, muscle-invasive bladder cancer, colorectal cancer, and hepatocellular carcinoma—demonstrated Signatera’s effectiveness in detecting MRD and predicting relapse. These results underscore the test’s potential to enhance personalized treatment strategies and improve patient outcomes.
June 2023: Caris Life Sciences expanded its partnership with ConcertAI to create a first-in-class, prospectively matched clinico-genomic research platform. This collaboration combines Caris’ comprehensive molecular profiling with ConcertAI’s real-world data and AI technology, aiming to advance precision oncology research. The integrated platform is designed to facilitate novel insights for therapeutic development and clinical trial management, thereby accelerating the translation of research findings into clinical practice.
These developments highlight the growing importance of integrating genomic data with real-world evidence to drive advancements in cancer research and treatment, ultimately aiming to improve patient care and outcomes.
The global genomic data set for the real-world evidence (RWE) applications market is witnessing significant growth and innovation, driven by the convergence of advanced genomics, healthcare analytics, and digital technologies. This expansive market holds immense potential to revolutionize healthcare research, treatment, and outcomes by providing crucial insights into the genetic underpinnings of diseases and personalized patient care.