Market Overview:
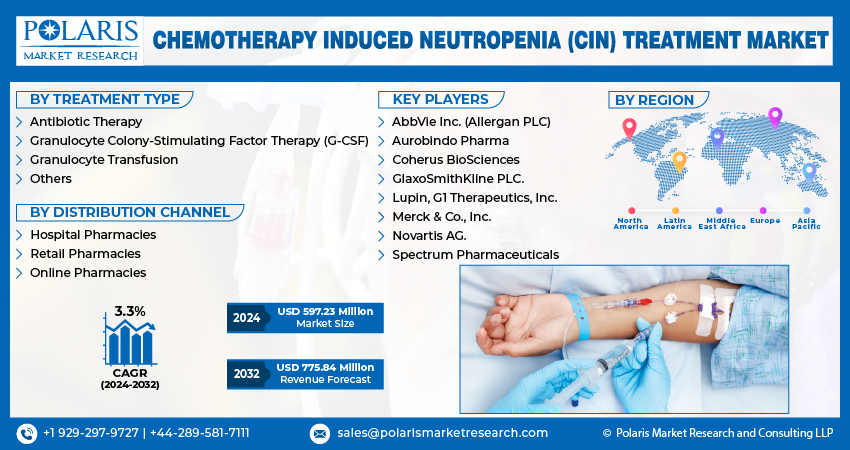
The global chemotherapy induced neutropenia treatment market size is expected to reach USD 775.84 million by 2032, exhibiting the CAGR of 3.3% during the forecast period.
What is Chemotherapy Induced Neutropenia (CIN) Treatment?
Chemotherapy-induced neutropenia is a significant outcome of cancer chemotherapies, several of which are massively myelosuppressive, and it does place a sizeable cost on a patient’s life with cancer. It can also cause hospitalization, where it can lead to sepsis and worsening of countless co-occurring conditions. In most cases, it can even cause death even though a supply of productive antibiotics. The resolution to utilize G-CSF agents as prime FN prevention is dependent on the chemotherapy regimen, particular probabilities of neutropenia progressions, and patient illness and treatments-connected probability elements.
Download Free Sample PDF Copy of the Report:
Leading Companies
The report provides a thorough inspection of both accepted and surfacing players within the market. It showcases a substantial list of leading firms categorized by the types of commodities they provide and several market-connected elements. Some of the notable payers in the market are enumerated as:
- AbbVie Inc. (Allergan PLC)
- Aurobindo Pharma
- Coherus BioSciences
- GlaxoSmithKline PLC.
- Lupin, G1 Therapeutics, Inc.
- Merck & Co., Inc.
- Novartis AG.
- Spectrum Pharmaceuticals
Growth Prospects and Trends
The chemotherapy induced neutropenia (CIN) treatment market is expanding due to the usage of chemotherapy as a treatment alternative for notable trends in contemporary healthcare. Many elements assist in a rise in cancer instances, involving population aging, environmental elements, lifestyle alternatives such as smoking and inadequate diet, and enhanced discernment procedures.
Moreover, research and development endeavours’ concentrate on advancing inventive treatment procedures targeted at enhancing the results for cancer patients experiencing chemotherapy. This involves the advancement of new drugs and biologics and encouraging care mediations, particularly those that aim to target neutropenia induced by chemotherapy.
Regional Insights
Chemotherapy Induced Neutropenia (CIN) Treatment Market, Regional Outlook (Revenue – USD Million, 2019-2032)
- North America
- Treatment Type Outlook
- Antibiotic Therapy
- Granulocyte Colony-Stimulating Factor Therapy (G-CSF)
- Granulocyte Transfusion
- Others
- Distribution Channel Outlook
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Treatment Type Outlook
- Europe
- Treatment Type Outlook
- Antibiotic Therapy
- Granulocyte Colony-Stimulating Factor Therapy (G-CSF)
- Granulocyte Transfusion
- Others
- Distribution Channel Outlook
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Treatment Type Outlook
- Asia Pacific
- Treatment Type Outlook
- Antibiotic Therapy
- Granulocyte Colony-Stimulating Factor Therapy (G-CSF)
- Granulocyte Transfusion
- Others
- Distribution Channel Outlook
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Treatment Type Outlook
- Latin America
- Treatment Type Outlook
- Antibiotic Therapy
- Granulocyte Colony-Stimulating Factor Therapy (G-CSF)
- Granulocyte Transfusion
- Others
- Distribution Channel Outlook
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Treatment Type Outlook
- North America makes up the majority of the market. This is primarily because of a well-advanced healthcare framework with progressive medical spaces, an approach to modern technologies, and an extremely adept workforce. This framework eases the diagnostics, treatment, and handling of cancer and its intricacies, including chemotherapy-induced neutropenia.
- The APAC market is expected to grow at a faster CAGR throughout the projection period. With a rapidly augmenting population, the region encounters growing cases of cancer credited to lifestyle alterations and maturing demographics.