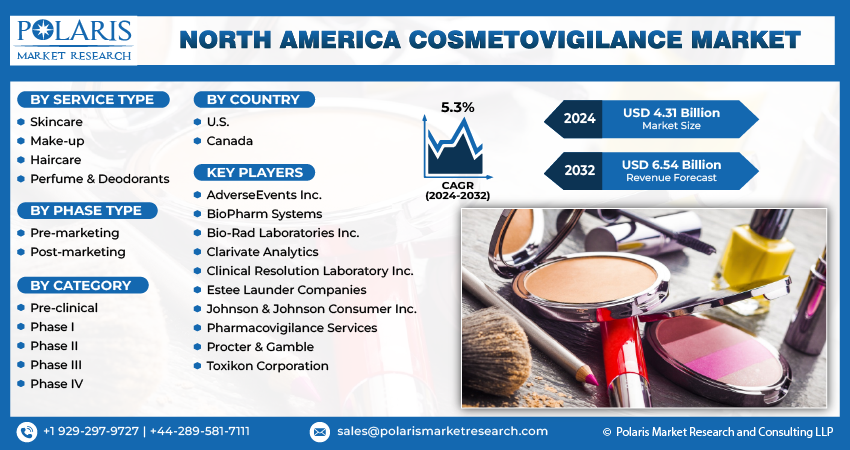
The North America cosmetovigilance market size is expected to reach USD 6.54 billion by 2032, exhibiting the CAGR of 5.3% during the forecast period.
The North America cosmetovigilance market has emerged as a critical segment within the broader cosmetics industry. With increasing consumer awareness about product safety and regulatory scrutiny, cosmetovigilance plays a pivotal role in monitoring, assessing, and preventing adverse effects related to cosmetic products. This growing focus on product safety and compliance has positioned cosmetovigilance as a cornerstone for both manufacturers and regulators in ensuring consumer trust and satisfaction.
𝐆𝐞𝐭 𝐄𝐱𝐜𝐥𝐮𝐬𝐢𝐯𝐞 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐚𝐠𝐞𝐬 𝐨𝐟 𝐓𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭:
Market’s Growth Drivers
Several factors are driving the growth of the North America cosmetovigilance market:
- Rising Consumer Awareness: Consumers are becoming more informed about the safety of the cosmetics they use, leading to heightened demand for transparency and accountability from manufacturers. This awareness drives companies to adopt robust cosmetovigilance practices.
- Stringent Regulatory Frameworks: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and Health Canada have implemented rigorous guidelines to ensure product safety. Non-compliance can result in severe penalties, motivating manufacturers to prioritize cosmetovigilance.
- Increase in Cosmetic Product Innovations: The introduction of novel cosmetic products, including organic and vegan options, necessitates comprehensive safety monitoring to mitigate potential risks.
- Growing Incidence of Adverse Reactions: Reports of adverse reactions to cosmetic products have surged, prompting manufacturers to enhance surveillance and reporting mechanisms.
- Digital Transformation in Reporting Systems: Advanced technologies like Artificial Intelligence (AI) and machine learning are revolutionizing adverse event reporting, making data collection and analysis more efficient.
Some of the major players operating in the global market include:
- AdverseEvents Inc.
- BioPharm Systems
- Bio-Rad Laboratories Inc.
- Clarivate Analytics
- Clinical Resolution Laboratory Inc.
- Estee Launder Companies
- Johnson & Johnson Consumer Inc.
- Pharmacovigilance Services
- Procter & Gamble
- Toxikon Corporation
Key Trends in the Market
The North America cosmetovigilance market is witnessing several notable trends:
- Shift Towards Proactive Monitoring: Companies are increasingly adopting proactive measures, such as pre-market testing and real-time monitoring, to identify and address potential risks early.
- Collaborations and Partnerships: Manufacturers are partnering with regulatory agencies and third-party organizations to improve compliance and streamline reporting processes.
- Emphasis on Natural and Organic Products: The rising demand for natural and organic cosmetics has led to the development of specialized safety protocols tailored to these products.
- Integration of Advanced Technologies: Tools like AI-powered chatbots and data analytics platforms are being utilized to handle customer complaints and adverse event reports effectively.
- Focus on Consumer Education: Many brands are investing in educating consumers about the safe use of their products, fostering trust and loyalty.
Report Segmentation
The research report categorizes the market into various segments and sub-segments. The primary segments covered in the study include service type, phase type, category. The splitting of the market into various groups enables businesses to understand market preferences and trends better.
North America Cosmetovigilance Market, Service Type Outlook (Revenue – USD Billion, 2019-2032)
- Skincare
- Make-up
- Haircare
- Perfume & Deodorants
North America Cosmetovigilance Market, Phase Type Outlook (Revenue – USD Billion, 2019-2032)
- Pre-marketing
- Post-marketing
North America Cosmetovigilance Market, Category Outlook (Revenue – USD Billion, 2019-2032)
- Foldable
- Non-foldable
Research Scope
The research scope of the North America cosmetovigilance market encompasses:
- Adverse Event Analysis: A detailed evaluation of reported adverse effects associated with various cosmetic products.
- Regulatory Impact Assessment: Analysis of the implications of changing regulations on market dynamics.
- Market Segmentation Studies: Insights into the market based on product type, end-user, and reporting mechanisms.
- Technological Advancements: Exploration of emerging technologies and their role in enhancing cosmetovigilance practices.
- Consumer Behavior Analysis: Examination of consumer preferences and their impact on the adoption of cosmetovigilance systems.
Future Outlook
The future of the North America cosmetovigilance market looks promising, driven by technological advancements, stricter regulations, and evolving consumer preferences. Key predictions include:
- Wider Adoption of AI and Automation: These technologies will further streamline data collection, analysis, and reporting, enhancing the overall efficiency of cosmetovigilance systems.
- Increased Collaboration: Greater collaboration between industry players, regulatory bodies, and research institutions is expected to foster innovation and compliance.
- Enhanced Product Safety Standards: Companies will likely adopt more rigorous safety protocols to maintain their market reputation and consumer trust.
- Expansion of Market Coverage: With the continuous introduction of innovative cosmetics, the scope of cosmetovigilance will expand to encompass diverse product categories.
- Growth in Personalized Cosmetics: The rise of personalized cosmetic products will demand customized safety monitoring practices.
Recent Developments:
March 2023:
ClinChoice Medical Development completed the acquisition of CROMSOURCE, a contract research organization (CRO) with seven subsidiaries in the United States. This strategic acquisition enhances ClinChoice’s service offerings across various domains, including pharmacovigilance, cosmetovigilance, toxicology, and other critical areas, bolstering its position as a comprehensive provider of clinical research solutions.