Mordor Intelligence has published a new report on the Preclinical CRO Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
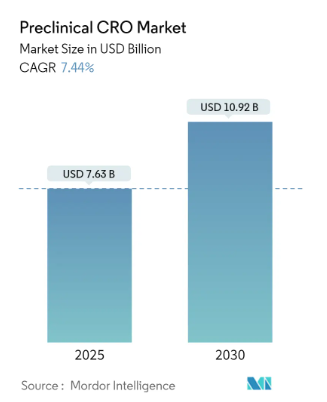
The global Preclinical Contract Research Organization (CRO) market is poised for significant growth, with projections indicating an increase from USD 7.63 billion in 2025 to USD 10.92 billion by 2030, reflecting a compound annual growth rate (CAGR) of 7.44% during this period. This expansion is largely attributed to escalating research and development (R&D) expenditures, a surge in the number of drugs undergoing preclinical trials, and a heightened demand for medications among patients with chronic illnesses.
Key Trends
Escalating R&D Expenditures
Pharmaceutical and biotechnology companies are substantially increasing their R&D investments to develop innovative treatments for chronic diseases. This financial commitment encompasses comprehensive drug discovery initiatives, including the execution of preclinical studies to assess the safety and efficacy of potential therapeutics. The growing focus on personalized medicine and targeted therapies further propels the demand for preclinical CRO services, as companies seek specialized expertise to navigate complex regulatory landscapes and expedite time-to-market.
Surge in Preclinical Trials
The pipeline of drugs in preclinical development has expanded considerably, driven by advancements in biotechnology and a deeper understanding of disease pathophysiology. This increase necessitates extensive preclinical testing to ensure candidate compounds meet safety and efficacy standards before progressing to clinical trials. Consequently, there is a heightened reliance on preclinical CROs to provide the necessary infrastructure, technical capabilities, and regulatory knowledge to conduct these studies efficiently.
Rising Demand for Chronic Disease Treatments
The global prevalence of chronic diseases such as diabetes, cardiovascular disorders, and cancer is on the rise, leading to an increased demand for effective therapeutic interventions. This trend drives pharmaceutical companies to accelerate the development of new drugs, thereby amplifying the need for preclinical CRO services to conduct requisite safety and efficacy evaluations. Additionally, the aging population and lifestyle-related health issues contribute to the sustained demand for chronic disease treatments, further bolstering market growth.
Report Overview: https://www.mordorintelligence.com/industry-reports/precilinical-cro-market
Market Segmentation
By Service
- Toxicology Testing: Critical for assessing the potential adverse effects of new chemical entities, toxicology testing services are essential to ensure patient safety and regulatory compliance.
- Bioanalysis and Drug Metabolism and Pharmacokinetics (DMPK) Studies: These services evaluate the absorption, distribution, metabolism, and excretion (ADME) properties of drug candidates, providing insights into their pharmacological profiles.
- Safety Pharmacology: Focused on identifying potential undesirable pharmacodynamic effects of substances on physiological functions, particularly the cardiovascular, respiratory, and central nervous systems.
- Other Services: Including chemistry, manufacturing, and control (CMC) studies, as well as regulatory consulting services.
By Mode Type
- Patient-Derived Organoid (PDO) Models: These models offer a more accurate representation of human biology, enhancing the predictive validity of preclinical studies.
- Patient-Derived Xenograft (PDX) Models: Utilized for in vivo studies, PDX models involve implanting human tumor tissues into immunodeficient mice, aiding in oncology drug development.
By End Users
- Biopharmaceutical Companies: Major consumers of preclinical CRO services, leveraging external expertise to streamline drug development processes.
- Research Institutes and Universities: Engage CROs for specialized preclinical studies to support academic research and translational medicine initiatives.
- Other End Users: Including government agencies and nonprofit organizations involved in drug discovery and development.
By Geography
- North America: Currently the largest market, attributed to robust R&D infrastructure and substantial healthcare expenditures.
- Europe: A significant market with a strong pharmaceutical industry presence and supportive regulatory frameworks.
- Asia-Pacific: Anticipated to be the fastest-growing region, driven by increasing R&D activities, favorable government initiatives, and a burgeoning biotechnology sector.
- Middle East and Africa: Emerging markets with growing investments in healthcare infrastructure and research capabilities.
- South America: Experiencing gradual growth due to expanding pharmaceutical industries and increasing focus on drug development.
Get a Customized Report Tailored to Your Requirements. – https://www.mordorintelligence.com/market-analysis/biotechnology
Key Players
The preclinical CRO market is characterized by low market concentration, with several key players contributing to its competitive landscape. Notable companies include:
- Charles River Laboratories: A leading full-service preclinical CRO offering a comprehensive portfolio of services, including toxicology, pathology, and pharmacology studies.
- Labcorp Drug Development: Provides extensive preclinical services, encompassing bioanalytical testing, DMPK studies, and regulatory support.
- Eurofins Scientific: Offers a wide range of preclinical testing services, including safety pharmacology, genetic toxicology, and bioanalysis.
- WuXi AppTec: A prominent CRO with capabilities in preclinical development, offering services such as toxicology testing, bioanalytical services, and pharmacokinetic studies.
- Medpace: Specializes in providing comprehensive preclinical and clinical development services, with expertise in various therapeutic areas.
These companies are actively engaged in strategic initiatives such as mergers and acquisitions, partnerships, and service portfolio expansions to enhance their market presence and meet the evolving demands of the pharmaceutical and biotechnology industries.
Conclusion
The preclinical CRO market is on a trajectory of sustained growth, driven by increased R&D expenditures, a burgeoning pipeline of drugs in preclinical trials, and a rising demand for treatments targeting chronic diseases. Advancements in preclinical models, such as PDO and PDX models, are enhancing the predictive accuracy of preclinical studies, thereby improving the efficiency of drug development processes. As the industry continues to evolve, preclinical CROs will play a pivotal role in facilitating the development of safe and effective therapeutics, ultimately contributing to improved patient outcomes globally.
Industry Related Reports
Mordor Intelligence is a trusted partner for businesses seeking comprehensive and actionable market intelligence. Our global reach, expert team, and tailored solutions empower organizations and individuals to make informed decisions, navigate complex markets, and achieve their strategic goals.
With a team of over 550 domain experts and on-ground specialists spanning 150+ countries, Mordor Intelligence possesses a unique understanding of the global business landscape. This expertise translates into comprehensive syndicated and custom research reports covering a wide spectrum of industries, including aerospace & defense, agriculture, animal nutrition and wellness, automation, automotive, chemicals & materials, consumer goods & services, electronics, energy & power, financial services, food & beverages, healthcare, hospitality & tourism, information & communications technology, investment opportunities, and logistics.
media@mordorintelligence.com
https://www.mordorintelligence.com/