The Europe Laboratory Developed Tests Market is a growing segment within the broader diagnostic testing industry. LDTs are tests that are designed, manufactured, and used within a single laboratory without being marketed or sold for commercial use. These tests are typically developed to address specific diagnostic needs or research questions that cannot be met by commercially available tests.
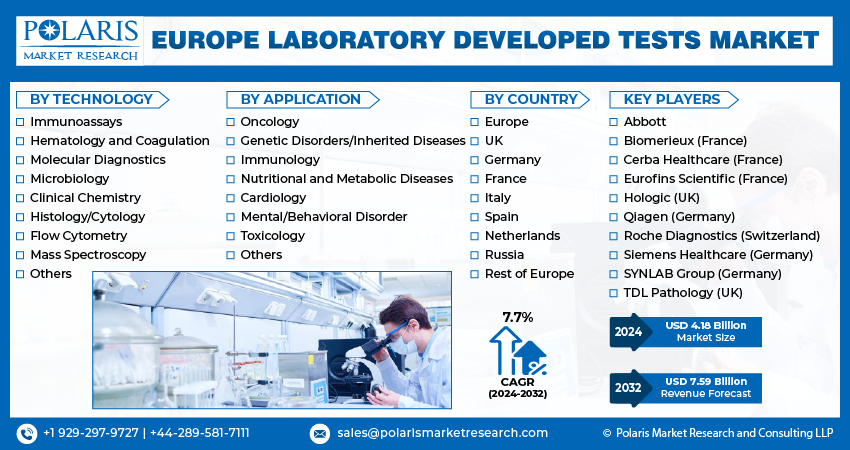
The Europe laboratory developed tests market size is expected to reach USD 7.59 billion by 2032, exhibiting the CAGR of 7.7% during the forecast period.
Market Growth Drivers
- Rising Demand for Personalized Medicine
Personalized medicine, which tailors treatment based on individual genetic profiles, has led to increased demand for LDTs that can offer precise and individualized diagnostic results. - Technological Advancements in Molecular Diagnostics
The rise of technologies such as next-generation sequencing (NGS), polymerase chain reaction (PCR), and CRISPR has enabled the development of more accurate and specialized LDTs. These technologies are particularly relevant for genetic testing, infectious diseases, and cancer diagnostics. - Increased Prevalence of Chronic Diseases
The rising prevalence of chronic diseases, such as cancer, diabetes, cardiovascular conditions, and genetic disorders, has driven the need for specialized tests to aid in diagnosis and disease management, boosting demand for LDTs.
Some of the major players operating in the global market include:
- Abbott
- Biomerieux (France)
- Cerba Healthcare (France)
- Eurofins Scientific (France)
- Hologic (UK)
- Qiagen (Germany)
- Roche Diagnostics (Switzerland)
- Siemens Healthcare (Germany)
- SYNLAB Group (Germany)
- TDL Pathology (UK)
Key Trends
- Increasing Use of Genetic Testing
Genetic testing through LDTs is growing rapidly, particularly for oncology, rare genetic disorders, and pharmacogenomics. LDTs provide customized genetic testing that can inform treatment decisions based on genetic markers. - Integration with Next-Generation Sequencing (NGS)
NGS technology has become a key driver of the LDT market, allowing for high-throughput, accurate testing for a wide range of conditions, particularly in oncology and hereditary diseases. - Regulatory Changes and Challenges
The regulatory environment for LDTs in Europe is evolving. The European Union (EU) Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced stricter guidelines for LDTs, which could impact market growth and product availability.
Recent Developments in the Industry
In October 2023, PathAI Diagnostics launched the world’s first AI-assisted laboratory-developed test designed for histologic scoring and staging of metabolic dysfunction-associated liver disease. This innovative tool employs an AI algorithm that has been shown to significantly reduce both inter- and intra-operator variability in CRN (Clinical Research Network) scoring.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/europe-laboratory-developed-tests-market
Challenges
- Regulatory Uncertainty
The lack of a unified regulatory framework for LDTs in Europe creates uncertainty and challenges for market players, especially with the introduction of the EU IVDR and MDR regulations. - Reimbursement Issues
Obtaining reimbursement for LDTs can be challenging, especially for new and unproven tests, which can limit adoption in certain healthcare systems. - Complexity in Test Validation
Developing and validating LDTs is a complex and resource-intensive process, requiring substantial investment in technology and expertise.
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐥 𝐎𝐯𝐞𝐫𝐯𝐢𝐞𝐰:
The research report categorizes the market into various segments and sub-segments. The primary segments covered in the study include type, application, end use and region. The splitting of the market into various groups enables businesses to understand market preferences and trends better. Also, stakeholders can develop products/services that align with the diverse needs of consumers in the industry. Besides, the research study includes a thorough examination of all the major sub-segments in the market.
Europe Laboratory Developed Tests, Technology Outlook (Revenue – USD Billion, 2019 – 2032)
- Immunoassays
- Hematology and Coagulation
- Molecular Diagnostics
- Microbiology
- Clinical Chemistry
- Histology/Cytology
- Flow Cytometry
- Mass Spectroscopy
- Others
Europe Laboratory Developed Tests, Application Outlook (Revenue – USD Billion, 2019 – 2032)
- Oncology
- Genetic Disorders/Inherited Diseases
- Immunology
- Nutritional and Metabolic Diseases
- Cardiology
- Mental/Behavioral Disorder
- Toxicology
- Others
The Europe Laboratory Developed Tests Market is poised for growth, driven by advancements in personalized medicine, increasing demand for genetic and molecular diagnostics, and the shift toward value-based healthcare. While regulatory challenges and reimbursement issues exist, the market presents significant opportunities for innovation, particularly in specialized and rare disease testing. With technological advancements in diagnostics and the increasing integration of LDTs into clinical and research settings, the European LDT market is set to continue expanding in the coming years.
More Trending Latest Reports By Polaris Market Research:
Aircraft Health Monitoring System Market
Hardware Security Modules Market