The Real World Evidence (RWE) Solutions Market is rapidly expanding, driven by the growing emphasis on evidence-based decision-making in healthcare. Real World Evidence refers to the clinical evidence derived from the analysis of real-world data (RWD), such as electronic health records (EHR), insurance claims data, patient registries, and data from wearable devices. RWE solutions provide valuable insights into the effectiveness, safety, and cost-effectiveness of medical treatments in routine clinical practice, outside the controlled environment of clinical trials.
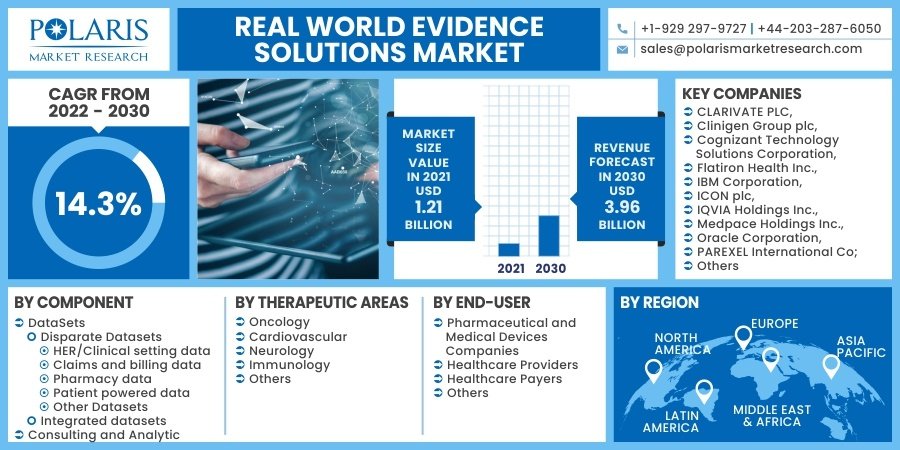
The global real world evidence solutions market size is expected to reach USD 3.96 billion by 2030, expected to grow at a CAGR of 14.3% during the forecast period.
Market Growth Drivers
- Increased Demand for Personalized Medicine
Personalized treatment approaches require data-driven insights from real-world settings to tailor therapies to individual patients. RWE provides the necessary data for these personalized approaches. - Shift Toward Value-Based Healthcare
Healthcare systems are increasingly moving toward value-based models, which require the evaluation of patient outcomes and treatment effectiveness. RWE solutions are crucial for assessing the real-world performance of treatments in diverse populations. - Regulatory Pressures and Requirements
Regulatory agencies, such as the U.S. FDA and European Medicines Agency (EMA), are increasingly relying on RWE for drug approval, post-market surveillance, and assessing treatment safety and efficacy in real-world settings.
Some of the major players operating in the global market include:
- CLARIVATE PLC
- Clinigen Group plc
- Cognizant Technology Solutions Corporation
- Flatiron Health Inc.
- IBM Corporation
- ICON plc
- IQVIA Holdings Inc.
- Medpace Holdings Inc.
- Oracle Corporation
Key Trends
- Integration of Artificial Intelligence and Machine Learning
The use of AI and machine learning in RWE solutions is gaining traction. These technologies allow for the automation of data analysis, improving the accuracy and speed of decision-making processes. - Collaboration Between Healthcare Stakeholders
Increasing partnerships between pharmaceutical companies, healthcare providers, payers, and regulatory bodies are leading to the pooling of data and expertise for more effective RWE analyses. - Focus on Predictive Analytics
Predictive analytics powered by real-world data are helping healthcare providers and pharmaceutical companies forecast outcomes, such as treatment efficacy, patient progression, and potential adverse events.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/real-world-evidence-solutions-market
Challenges
- Data Privacy and Security Concerns
With the vast amount of sensitive patient data involved, maintaining data privacy and security remains a significant challenge for RWE providers. - Data Quality and Standardization
The diversity of data sources can result in inconsistent data quality, making it difficult to draw reliable conclusions. Standardizing data collection and analysis methods is a key challenge. - Regulatory Uncertainty
The regulatory environment for RWE is still evolving, with varying requirements across regions. Companies must navigate complex regulatory landscapes to ensure compliance.
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐥 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬:
The research study includes segmental analysis that divides the market into distinct groups or segments based on common characteristics. With market segmentation, businesses can identify specific customer groups that are more likely to be interested in specific products or services. Also, it enables these businesses to focus their marketing efforts and resources more efficiently, leading to higher conversion rates and improved return on investment. Furthermore, segmentation analysis helps companies develop personalized products or services, which can result in increased customer loyalty and improved customer satisfaction.
Real World Evidence Solutions, Component Outlook (Revenue – USD Billion, 2018 – 2030)
- DataSets
- Disparate Datasets
- HER/Clinical setting data
- Claims and billing data
- Pharmacy data
- Patient powered data
- Other Datasets
- Integrated datasets
- Disparate Datasets
- Consulting and Analytics
Real World Evidence Solutions, Therapeutic Areas Outlook (Revenue – USD Billion, 2018 – 2030)
- Oncology
- Cardiovascular
- Neurology
- Immunology
- Others
Real World Evidence Solutions, End-User Outlook (Revenue – USD Billion, 2018 – 2030)
- Pharmaceutical and Medical Devices Companies
- Healthcare Providers
- Healthcare Payers
- Other End Users
The Real World Evidence Solutions Market is poised for significant growth, driven by the increasing adoption of personalized medicine, value-based healthcare, and the growing demand for data-driven decision-making. As the healthcare landscape becomes more data-centric, RWE solutions will play an essential role in improving patient outcomes, optimizing treatment effectiveness, and ensuring cost-efficient care. However, addressing challenges related to data privacy, integration, and regulatory compliance will be crucial for unlocking the full potential of this market.
More Trending Latest Reports By Polaris Market Research:
Circulating Tumor Cells Market
Cryo-electron Microscopy Market
North America Robot Operating System Market
AI Model Risk Management Market