The global regulatory affairs outsourcing market is experiencing significant growth, driven by the increasing complexity of the regulatory landscape, the growing need for specialized expertise, and the rising pressure to accelerate drug development timelines.
Market Overview:
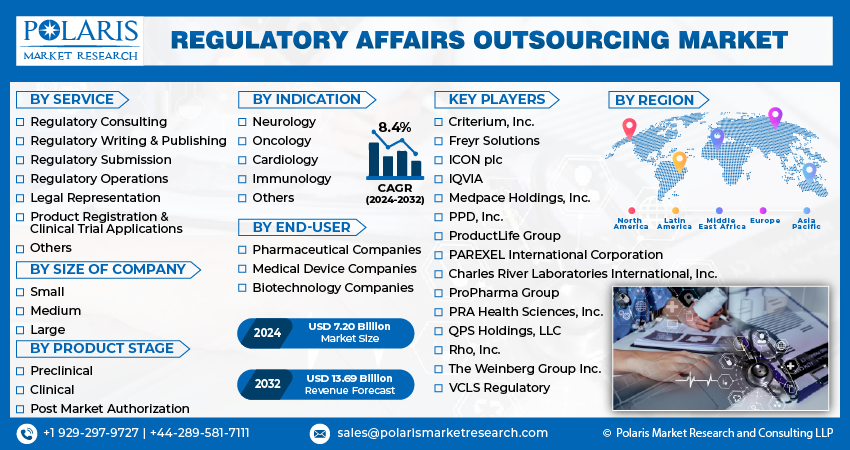
The global regulatory affairs outsourcing market size was valued at USD 6.66 billion in 2023 and is anticipated to grow from USD 7.20 billion in 2024 to USD 13.69 billion by 2032, exhibiting a CAGR of 8.4% during the forecast period. This growth reflects the increasing reliance of pharmaceutical and biotechnology companies on specialized regulatory affairs expertise to navigate the complex regulatory hurdles associated with drug development and commercialization.
Some of the major players operating in the global market include:
- Charles River Laboratories International, Inc.
- Criterium, Inc.
- Freyr Solutions
- ICON plc
- IQVIA
- Medpace Holdings, Inc.
- PAREXEL International Corporation
- PPD, Inc.
- ProductLife Group
- ProPharma Group
- PRA Health Sciences, Inc.
- QPS Holdings, LLC
- Rho, Inc.
- The Weinberg Group Inc.
- VCLS Regulatory
Key Market Drivers:
- Increasing Regulatory Complexity: The increasing complexity of global regulatory requirements, including those related to drug safety, efficacy, and quality, is driving the demand for specialized regulatory expertise.
- Accelerated Drug Development: The pressure to accelerate drug development timelines and bring new therapies to market faster is driving the need for efficient and streamlined regulatory processes.
- Focus on Core Competencies: Pharmaceutical and biotechnology companies are increasingly focusing on their core competencies, such as research and development, and outsourcing non-core functions such as regulatory affairs to specialized CROs.
- Advancements in Technology: Advancements in technology, such as the use of electronic submissions and data analytics, are improving the efficiency and effectiveness of regulatory processes.
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐥 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬:
Regulatory Affairs Outsourcing Market, Service Outlook (Revenue, USD Billion, 2019-2032)
- Regulatory Consulting
- Regulatory Writing & Publishing
- Regulatory Submission
- Regulatory Operations
- Legal Representation
- Product Registration & Clinical Trial Applications
- Others
Regulatory Affairs Outsourcing Market, Size of company Outlook (Revenue, USD Billion, 2019-2032)
- Small
- Medium
- Large
Regulatory Affairs Outsourcing Market, Indication Outlook (Revenue, USD Billion, 2019-2032)
- Neurology
- Oncology
- Cardiology
- Immunology
- Others
Regulatory Affairs Outsourcing Market, Product Stage Outlook (Revenue, USD Billion, 2019-2032)
- Preclinical
- Clinical
- Post Market Authorization
Regulatory Affairs Outsourcing Market, End-User Outlook (Revenue, USD Billion, 2019-2032)
- Pharmaceutical Companies
- Medical Device Companies
- Biotechnology Companies
The research study includes segmental analysis that divides the market into distinct groups or segments based on common characteristics. With market segmentation, businesses can identify specific customer groups that are more likely to be interested in specific products or services. Also, it enables these businesses to focus their marketing efforts and resources more efficiently, leading to higher conversion rates and improved return on investment. Furthermore, segmentation analysis helps companies develop personalized products or services, which can result in increased customer loyalty and improved customer satisfaction.
Recent Developments:
- ProductLife Group Expands Capabilities: In May 2023, ProductLife Group expanded its capabilities by merging with Cilatus BioPharma Consulting and Cilatus Manufacturing Services. This merger enhances PLG’s ability to provide comprehensive product development and regulatory support services to its clients.
- VCLS Collaborates with EC Innovations: In April 2022, VCLS entered into a collaboration with EC Innovations to enhance its translation services. This partnership improves the quality and efficiency of regulatory submissions by ensuring accurate and culturally sensitive translations of regulatory documents.
Table of Contents:
- Global Regulatory Affairs Outsourcing Market Insights
4.1. Regulatory Affairs Outsourcing – Industry Snapshot
4.2. Regulatory Affairs Outsourcing Market Dynamics
4.2.1. Drivers and Opportunities
4.2.1.1. Increased fixed costs is projected to spur the product demand
4.2.1.2. Rising number of clinical trials is expected to drive regulatory affairs outsourcing market growth
4.2.2. Restraints and Challenges
4.2.2.1. Increasing data security risks is likely to impede the regulatory affairs outsourcing market growth opportunities
4.3. Porter’s Five Forces Analysis
4.3.1. Bargaining Power of Suppliers (Moderate)
4.3.2. Threats of New Entrants: (Low)
4.3.3. Bargaining Power of Buyers (Moderate)
4.3.4. Threat of Substitute (Moderate)
4.3.5. Rivalry among existing firms (High)
4.4. PESTLE Analysis
4.5. Regulatory Affairs Outsourcing Industry Trends
4.6. COVID-19 Impact Analysis
5. Global Regulatory Affairs Outsourcing Market, by Indication
5.1. Key Findings
5.2. Introduction
5.2.1. Global Regulatory Affairs Outsourcing Market, by Indication, 2019-2032 (USD Billion)
5.3. Neurology
5.3.1. Global Regulatory Affairs Outsourcing Market, by Neurology, by Region, 2019-2032 (USD Billion)
5.4. Oncology
5.4.1. Global Regulatory Affairs Outsourcing Market, by Oncology, by Region, 2019-2032 (USD Billion)
5.5. Cardiology
5.5.1. Global Regulatory Affairs Outsourcing Market, by Cardiology, by Region, 2019-2032 (USD Billion)
5.6. Immunology
5.6.1. Global Regulatory Affairs Outsourcing Market, by Immunology, by Region, 2019-2032 (USD Billion)
5.7. Others
5.7.1. Global Regulatory Affairs Outsourcing Market, by Others, by Region, 2019-2032 (USD Billion)
6. Global Regulatory Affairs Outsourcing Market, by Product Stage
6.1. Key Findings
6.2. Introduction
6.2.1. Global Regulatory Affairs Outsourcing Market, by Product Stage, 2019-2032 (USD Billion)
6.3. Preclinical
6.3.1. Global Regulatory Affairs Outsourcing Market, by Preclinical, by Region, 2019-2032 (USD Billion)
6.4. Clinical
6.4.1. Global Regulatory Affairs Outsourcing Market, by Clinical, by Region, 2019-2032 (USD Billion)
6.5. Post Market Authorization
6.5.1. Global Regulatory Affairs Outsourcing Market, by Post Market Authorization, by Region, 2019-2032 (USD Billion)
Conclusion:
The global regulatory affairs outsourcing market is poised for continued growth, driven by the increasing complexity of the regulatory landscape, the growing need for specialized expertise, and the increasing pressure to accelerate drug development. Continued innovation in regulatory science and the development of more efficient and streamlined regulatory processes will be crucial for the future of this dynamic market.
More Trending Latest Reports By Polaris Market Research:
U.S. Durable Medical Equipment Market
Europe Veterinary Clinical Trials Market
Internet Of Things (Iot) In Healthcare Market