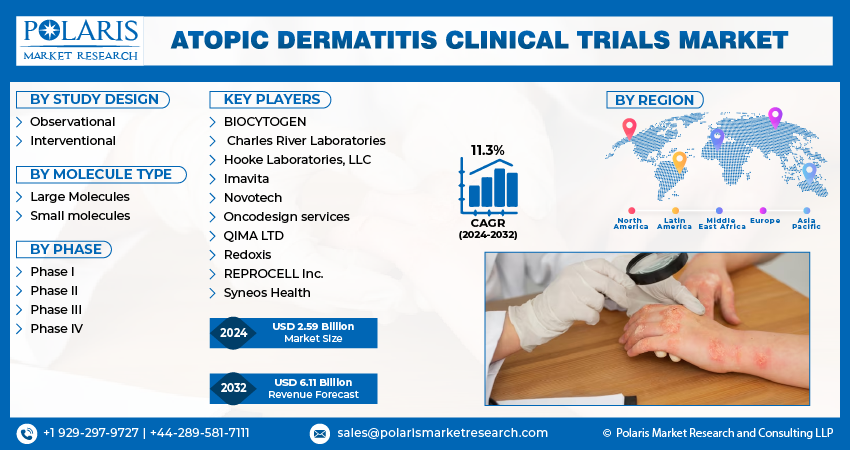
The global atopic dermatitis (AD) clinical trials market is experiencing substantial growth, driven by the increasing prevalence of this chronic skin condition and the ongoing development of novel therapies. With a projected market size of USD 6.11 billion by 2032 and a robust CAGR of 11.3%, the market reflects a significant investment in research and development aimed at improving treatment options for individuals with AD.
Market Overview
Atopic dermatitis, also known as eczema, is a chronic inflammatory skin condition characterized by itchy, red, and inflamed skin. While its exact cause remains unknown, it is believed to involve a complex interplay of genetic, environmental, and immunological factors. The rising prevalence of AD, particularly in developed countries, has fueled the demand for effective and safe treatments.
Some of the major players operating in the global market include:
- BIOCYTOGEN
- Charles River Laboratories
- Hooke Laboratories, LLC
- Imavita
- Novotech
- Oncodesign services
- QIMA LTD
- Redoxis
- REPROCELL Inc.
- Syneos Health
Key Market Drivers
- Increasing Prevalence of Atopic Dermatitis: The rising incidence of AD across all age groups, including children, is a primary driver of market growth.
- Advancements in Therapeutics: Ongoing research and development of novel therapies, including biologics, targeted therapies, and topical medications, are driving innovation and expanding treatment options.
- Unmet Medical Needs: Despite existing treatment options, many individuals with AD still experience significant symptoms and have unmet medical needs. This drives the need for continued research and development of more effective and tolerable therapies.
- Growing Research and Development Investment: Pharmaceutical companies and research institutions are investing heavily in AD research, fueling the growth of the clinical trials market.
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐥 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬:
Atopic Dermatitis Clinical Trials Market, Study Design Outlook (Revenue – USD Billion, 2019-2032)
- Observational
- Interventional
Atopic Dermatitis Clinical Trials Market, Molecule Type Outlook (Revenue – USD Billion, 2019-2032)
- Large Molecules
- Small molecules
Atopic Dermatitis Clinical Trials Market, Phase Outlook (Revenue – USD Billion, 2019-2032)
- Phase I
- Phase II
- Phase III
- Phase IV
The research study includes segmental analysis that divides the market into distinct groups or segments based on common characteristics. With market segmentation, businesses can identify specific customer groups that are more likely to be interested in specific products or services. Also, it enables these businesses to focus their marketing efforts and resources more efficiently, leading to higher conversion rates and improved return on investment. Furthermore, segmentation analysis helps companies develop personalized products or services, which can result in increased customer loyalty and improved customer satisfaction.
Growth Trends
- Focus on Biologics: Biologics, such as dupilumab and baricitinib, have demonstrated significant efficacy in treating moderate-to-severe AD and are expected to continue to play a major role in the market.
- Development of Novel Topical Therapies: Research and development of novel topical therapies, including topical JAK inhibitors and other targeted therapies, are ongoing and hold significant promise for improving treatment outcomes.
- Pediatric Focus: There is a growing focus on developing safe and effective treatments for children with AD, particularly those with severe disease.
- Precision Medicine: Advancements in personalized medicine, including the use of biomarkers and genetic testing, are enabling the development of more targeted and effective therapies.
Recent Developments
- Positive Results for VTAMA Cream: Positive results from the ADORING 1 trial for VTAMA (tapinarof) cream 1% in treating moderate-to-severe AD in adults and children as young as two years old represent a significant advancement in treatment options.
- Encouraging Results for Delgocitinib Cream: Positive results from the DELTA 1 trial for delgocitinib cream in treating chronic hand eczema (CHE) demonstrate the potential of topical JAK inhibitors for treating various forms of eczema.
- FDA Approval of CIBINQO: The FDA approval of CIBINQO (abrocitinib) for the treatment of moderate-to-severe AD in adults further expands treatment options for patients.
Challenges and Opportunities
- High Development Costs: The high costs associated with drug development and clinical trials can be a significant barrier to entry for many companies.
- Regulatory Challenges: Navigating the complex regulatory landscape for drug development and approval can be challenging.
- Patient Recruitment and Retention: Recruiting and retaining patients for clinical trials can be challenging, particularly for rare diseases.
Opportunities:
- Development of Novel Therapeutics: Continued research and development of novel therapies, including biologics, targeted therapies, and gene therapies, offer significant opportunities for market growth.
- Personalized Medicine: The development of personalized medicine approaches, including biomarker-driven therapies, offers the potential to improve treatment outcomes and reduce side effects.
- Digital Health Technologies: The integration of digital health technologies, such as telemedicine and remote patient monitoring, can improve the efficiency and effectiveness of clinical trials.
Table of Contents:
4. Global Atopic Dermatitis Clinical Trials Market Insights
4.1. Atopic Dermatitis Clinical Trials Market – Phase Snapshot
4.2. Atopic Dermatitis Clinical Trials Market Dynamics
4.2.1. Drivers and Opportunities
4.2.1.1. Increasing prevalence of atopic dermatitis
4.2.1.2. Enhancing comprehension of the mechanisms underlying diseases
4.2.2. Restraints and Challenges
4.2.2.1. Rigorous regulatory standards
4.3. Porter’s Five Forces Analysis
4.3.1. Bargaining Power of Suppliers (Moderate)
4.3.2. Threats of New Entrants: (Low)
4.3.3. Bargaining Power of Buyers (Moderate)
4.3.4. Threat of Substitute (Moderate)
4.3.5. Rivalry among existing firms (High)
4.4. PESTEL Analysis
4.5. Atopic Dermatitis Clinical Trials Market Phase Trends
4.6. Value Chain Analysis
4.7. COVID-19 Impact Analysis
5. Global Atopic Dermatitis Clinical Trials Market, by Study Design
5.1. Key Findings
5.2. Introduction
5.2.1. Global Atopic Dermatitis Clinical Trials Market, by Study Design, 2019-2032 (USD Billion)
5.3. Observational
5.3.1. Global Atopic Dermatitis Clinical Trials Market, by Observational, by Region, 2019-2032 (USD Billion)
5.4. Interventional
5.4.1. Global Atopic Dermatitis Clinical Trials Market, by Interventional, by Region, 2019-2032 (USD Billion)
6. Global Atopic Dermatitis Clinical Trials Market, by Molecule Type
6.1. Key Findings
6.2. Introduction
6.2.1. Global Atopic Dermatitis Clinical Trials Market, by Molecule Type, 2019-2032 (USD Billion)
6.3. Large Molecules
6.3.1. Global Atopic Dermatitis Clinical Trials Market, by Large Molecules, by Region, 2019-2032 (USD Billion)
6.4. Small molecules
6.4.1. Global Atopic Dermatitis Clinical Trials Market, by Small molecules, by Region, 2019-2032 (USD Billion)
Conclusion
The atopic dermatitis clinical trials market is a dynamic and rapidly evolving landscape. Continued research and development, coupled with advancements in technology and personalized medicine, are expected to drive significant growth and improve treatment outcomes for individuals with this chronic skin condition.
More Trending Latest Reports By Polaris Market Research: