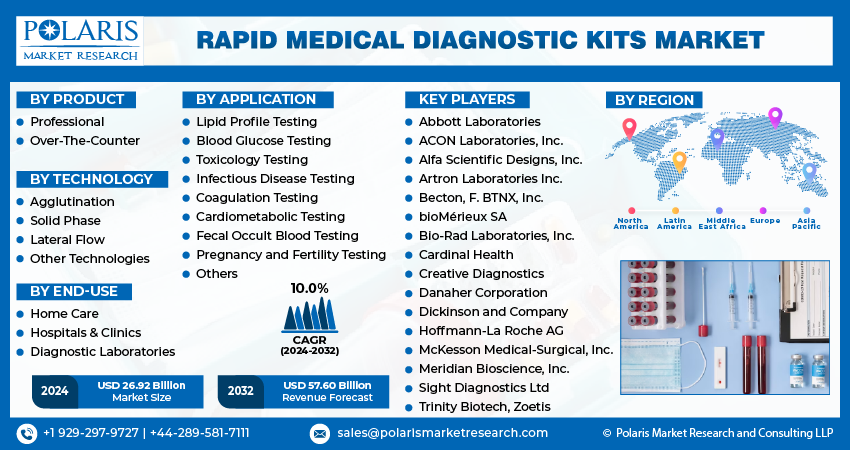
The global rapid medical diagnostic kits market is poised for significant growth, projected to reach USD 57.60 billion by 2032, exhibiting a robust compound annual growth rate (CAGR) of 10.0% during the forecast period. This surge reflects the increasing demand for faster, more convenient, and accessible diagnostic solutions in healthcare. This article delves into the key drivers, growth patterns, emerging trends, and opportunities within this dynamic and rapidly evolving market.
Market Overview
Rapid medical diagnostic kits, often referred to as point-of-care (POC) tests, are designed to provide rapid and accurate results for a variety of medical conditions. These kits typically involve simple procedures, often requiring only a small sample (e.g., blood, urine, saliva) and minimal equipment, making them suitable for use in various settings, including clinics, hospitals, and even at home. Key applications of rapid diagnostic kits include:
- Infectious Disease Diagnosis: Detection of infectious diseases such as influenza, strep throat, COVID-19, HIV, and sexually transmitted infections.
- Pregnancy Testing: Detection of pregnancy through the identification of human chorionic gonadotropin (hCG) hormone.
- Blood Glucose Monitoring: Monitoring blood sugar levels in individuals with diabetes.
- Cardiac Markers: Detection of cardiac biomarkers, such as troponin, to assess the risk of heart attack.
- Tumor Markers: Detection of tumor markers to aid in the diagnosis and monitoring of cancer.
Key Drivers of Market Growth
Several factors are contributing to the expansion of the rapid medical diagnostic kits market:
- Rising Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases, such as diabetes, cardiovascular diseases, and infectious diseases, is driving the demand for rapid and accurate diagnostic tools.
- Growing Emphasis on Early Disease Detection: The growing emphasis on early disease detection and preventive healthcare is driving the adoption of rapid diagnostic tests for early diagnosis and treatment initiation.
- Advancements in Technology: Continuous advancements in diagnostic technologies, such as the development of more sensitive and specific assays, improved sample preparation techniques, and integration of digital technologies, are enhancing the accuracy and reliability of rapid diagnostic tests.
- Point-of-Care Testing: The increasing demand for point-of-care testing, which allows for rapid diagnosis and treatment in various settings, including clinics, emergency departments, and even at home, is driving market growth.
- Global Health Concerns: Outbreaks of infectious diseases, such as pandemics, have highlighted the importance of rapid and accurate diagnostic testing for disease surveillance, outbreak control, and public health management.
Some of the major players operating in the global market include:
- Abbott Laboratories
- ACON Laboratories, Inc.
- Alfa Scientific Designs, Inc.
- Artron Laboratories Inc.
- Becton
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Cardinal Health
- Creative Diagnostics
- Danaher Corporation
- Dickinson and Company
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/rapid-medical-diagnostic-kits-market
Growth Trajectories
The rapid medical diagnostic kits market is expected to experience significant growth across different regions:
- North America: This region currently holds a significant market share due to well-established healthcare infrastructure, high adoption rates of advanced technologies, and a strong focus on patient care.
- Europe: The European market is expected to witness steady growth, driven by increasing healthcare expenditure, advancements in medical technology, and a focus on improving patient outcomes.
- Asia-Pacific: This region is poised for rapid growth, fueled by a large and growing population, increasing healthcare expenditure, and rising awareness of the benefits of rapid diagnostic tests.
Emerging Trends
Several key trends are shaping the future of the rapid medical diagnostic kits market:
- Multiplexing: The development of multiplex assays that can simultaneously detect multiple targets, such as multiple pathogens or biomarkers, is improving the efficiency and cost-effectiveness of diagnostic testing.
- Integration with Digital Health: The integration of rapid diagnostic tests with digital health platforms, such as smartphones and wearable devices, is enabling data collection, remote monitoring, and improved patient care.
- Artificial Intelligence (AI) and Machine Learning: The application of AI and machine learning algorithms is enabling more accurate and efficient data analysis, improving the interpretation of diagnostic results and facilitating personalized medicine.
- Point-of-Care Testing (POCT) Devices: The development of more sophisticated and user-friendly POCT devices is expanding the accessibility and convenience of rapid diagnostic testing.
- Focus on Home Testing: The increasing availability of over-the-counter (OTC) rapid diagnostic tests for conditions such as influenza and COVID-19 is expanding access to testing and empowering individuals to take control of their health.
Market Opportunities
The rapid medical diagnostic kits market offers several lucrative opportunities:
- Development of Novel Diagnostic Technologies: Research and development of novel diagnostic technologies, such as microfluidic devices, biosensors, and nanotechnology-based assays, can create new market avenues.
- Expansion into Emerging Markets: Expanding market presence in emerging economies with growing healthcare needs and increasing access to healthcare services can drive significant revenue growth.
- Focus on Personalized Medicine: Developing personalized diagnostic approaches, such as biomarker panels and genetic testing, can improve the accuracy and effectiveness of disease diagnosis and treatment.
- Strategic Partnerships: Collaborating with healthcare providers, technology companies, and research institutions can facilitate the development and commercialization of innovative diagnostic technologies.
Recent Developments
The rapid medical diagnostic kits market has witnessed several notable developments:
- Approval of New Diagnostic Tests: The approval of new and improved diagnostic tests, such as rapid COVID-19 tests and new tests for infectious diseases, is expanding the range of available diagnostic options.
- Advancements in Diagnostic Technologies: Advancements in diagnostic technologies, such as the development of more sensitive and specific assays and the integration of digital technologies, are improving the accuracy and efficiency of rapid diagnostic testing.
- Growing Adoption of Point-of-Care Testing: The increasing adoption of point-of-care testing in various settings, including clinics, emergency departments, and even at home, is expanding the accessibility and convenience of rapid diagnostic testing.
- Increased Investment in Research and Development: Increased investment in research and development activities is fueling the development of innovative diagnostic technologies and improving the overall quality of rapid diagnostic testing.
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐥 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬:
The research study includes segmental analysis that divides the market into distinct groups or segments based on common characteristics. With market segmentation, businesses can identify specific customer groups that are more likely to be interested in specific products or services. Also, it enables these businesses to focus their marketing efforts and resources more efficiently, leading to higher conversion rates and improved return on investment. Furthermore, segmentation analysis helps companies develop personalized products or services, which can result in increased customer loyalty and improved customer satisfaction.
Rapid Medical Diagnostic Kits, Product Outlook (Revenue – USD Billion, 2019 – 2032)
- Professional
- Over-The-Counter (OTC)
Rapid Medical Diagnostic Kits, Technology Outlook (Revenue – USD Billion, 2019 – 2032)
- Agglutination
- Solid Phase
- Lateral Flow
- Other Technologies
Rapid Medical Diagnostic Kits, Application Outlook (Revenue – USD Billion, 2019 – 2032)
- Lipid Profile Testing
- Blood Glucose Testing
- Toxicology Testing
- Infectious Disease Testing
- Coagulation Testing
- Cardiometabolic Testing
Rapid Medical Diagnostic Kits, End-Use Outlook (Revenue – USD Billion, 2019 – 2032)
- Home Care
- Hospitals & Clinics
- Diagnostic Laboratories s
Conclusion
The rapid medical diagnostic kits market is experiencing a period of significant growth, driven by advancements in technology, increasing demand for faster and more convenient diagnostic solutions, and the growing emphasis on early disease detection and preventive healthcare. By embracing innovation, focusing on patient needs, and addressing the evolving needs of the healthcare system, the rapid medical diagnostic kits market is poised for continued expansion, playing a crucial role in improving healthcare access, enhancing patient outcomes, and improving public health.
More Trending Latest Reports By Polaris Market Research:
Industrial Refrigeration Systems Market