Market Overview
The global medical device industry operates within a complex web of stringent regulatory requirements, patient safety protocols, and global quality standards. Central to these dynamics is the vital function of medical device complaint management—a system that helps manufacturers track, analyze, resolve, and report device-related issues to ensure product safety and regulatory compliance.
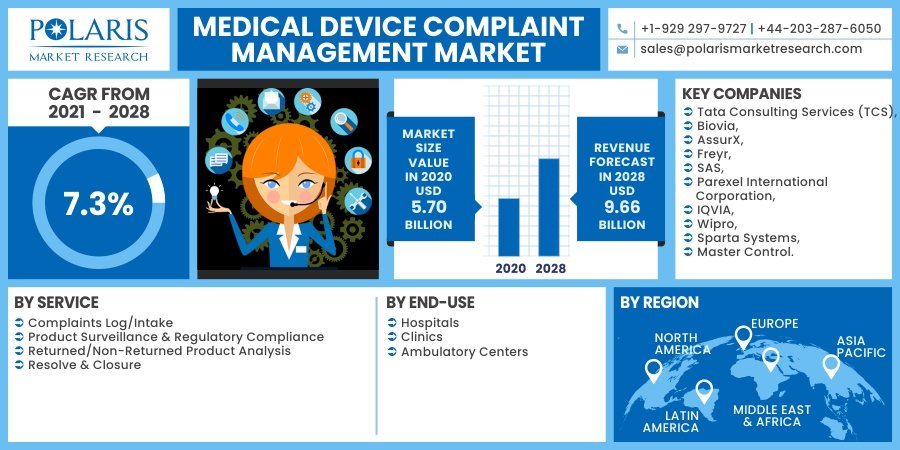
According to the research report published by Polaris Market Research, the global medical device complaint management market was valued at USD 5.70 billion in 2020 and is projected to expand at a compound annual growth rate (CAGR) of 7.3% during the forecast period. The growing demand for quality assurance, increasing regulatory scrutiny, the digitization of compliance processes, and the proliferation of medical devices across global markets are driving this growth.
This article provides an in-depth analysis of the medical device complaint management market, exploring its growth drivers, key trends, segmentation, and the evolving research landscape.
Market Growth Drivers
- Rising Regulatory Stringency and Global Compliance Norms
One of the most significant growth drivers for the complaint management market is the increasingly rigorous regulatory landscape. Regulatory bodies such as the U.S. FDA, European Medicines Agency (EMA), China’s NMPA, and others require manufacturers to establish robust post-market surveillance and complaint-handling processes. Non-compliance can result in recalls, warning letters, or product bans, making complaint management systems indispensable.
- Growing Medical Device Usage and Product Complexity
The expansion of the global medical device market—ranging from simple surgical tools to complex implantables and software-based devices—has increased the volume and complexity of device complaints. As more devices are adopted across hospitals, clinics, and homecare settings, manufacturers need agile systems to track field performance and respond promptly to adverse events.
- Emphasis on Patient Safety and Product Quality
Governments and healthcare providers worldwide are intensifying efforts to improve patient safety. Medical device complaints, whether they relate to malfunctions, labeling issues, or performance problems, pose a direct risk to patients. A robust complaint management framework allows for real-time incident monitoring, root cause analysis, and corrective action—reducing liability and improving outcomes.
- Integration of Digital and Cloud-Based Platforms
With the advent of digital health and smart manufacturing, complaint management systems are increasingly being deployed as cloud-based solutions. These platforms facilitate faster reporting, centralized data handling, cross-functional collaboration, and real-time analytics—all of which improve regulatory readiness and process efficiency.
- Rise in Medical Device Recalls and Adverse Event Reports
An uptick in global medical device recalls, fueled by product flaws, regulatory crackdowns, and heightened consumer awareness, has pushed manufacturers to implement structured complaint handling. According to the FDA, medical device recalls have increased significantly over the past decade—highlighting the need for transparent and automated complaint tracking systems.
Key Trends Shaping the Market
- AI-Powered Complaint Analytics
Artificial intelligence (AI) is being integrated into complaint management systems to enable predictive analytics, automated classification of complaints, and natural language processing for better understanding of patient feedback. These tools help reduce processing times, identify systemic issues, and streamline decision-making.
- Integration with Quality Management Systems (QMS)
Modern complaint management platforms are being integrated into broader Quality Management Systems (QMS), enabling end-to-end visibility from complaint logging to CAPA (Corrective and Preventive Actions). This seamless integration ensures traceability, compliance, and accelerated resolution timelines.
- Expansion of SaaS-Based Solutions
Software-as-a-Service (SaaS) is becoming the preferred deployment model for medical device complaint handling. These solutions are scalable, accessible, and cost-effective, allowing even small to medium-sized enterprises (SMEs) to maintain compliance without heavy infrastructure investments.
- Automated Reporting to Regulatory Authorities
Advanced platforms are offering built-in capabilities to auto-generate and submit required regulatory reports such as MDRs (Medical Device Reports), Vigilance Reports, and Field Safety Corrective Action (FSCA) documents to authorities like the FDA and EMA—reducing compliance risk and administrative burden.
- Globalization of Complaint Handling
With medical device manufacturers operating globally, complaint handling must account for language localization, regional regulations, and diverse patient feedback mechanisms. Platforms with multilingual support and regional workflow customization are gaining popularity.
Research Scope
The research scope of the global medical device complaint management market spans across:
- Market dynamics analysis including drivers, restraints, opportunities, and threats.
- Industry landscape with focus on technological evolution, regulatory impacts, and digitization.
- Competitive benchmarking across major vendors and emerging players.
- Geographic analysis to identify region-wise trends and adoption rates.
- Forecast modeling from historical data and predictive analytics for market estimation through 2028.
This comprehensive analysis is essential for manufacturers, regulatory consultants, software vendors, and investors to navigate the rapidly evolving compliance environment.
Market Segmentation
To understand the depth of the market, it is segmented based on component, deployment type, product type, end user, and geography.
- By Component
- Software: Includes complaint management platforms and modules integrated within enterprise resource planning (ERP) or QMS systems.
- Services: Encompasses implementation, consulting, maintenance, and training services provided by vendors.
Software holds a significant share due to the rising demand for automation and real-time analytics in complaint processing.
- By Deployment Type
- On-Premise: Traditional model with in-house server infrastructure; preferred by large enterprises with stringent data control needs.
- Cloud-Based: Fastest-growing segment due to ease of deployment, cost efficiency, and flexibility in remote access.
Cloud-based models are seeing increased adoption across small and mid-sized device manufacturers looking for scalable and agile compliance solutions.
- By Product Type
- Diagnostic Devices: Include imaging systems, IVD equipment, and monitoring tools.
- Therapeutic Devices: Cover implantable devices, infusion pumps, and surgical instruments.
- Others: Such as software as a medical device (SaMD) and combination products.
Complaint management systems must be tailored to the complexity and risk classification of each device category, as regulatory obligations vary accordingly.
- By End User
- Medical Device Manufacturers: Primary users responsible for complaint intake, triage, investigation, and reporting.
- Contract Manufacturing Organizations (CMOs): Outsourced service providers who need compliant systems to manage complaints and CAPAs.
- Third-Party Complaint Handling Services: Includes call centers, regulatory consultants, and software-as-a-service vendors managing complaint intake and response on behalf of device companies.
Manufacturers remain the largest segment, but outsourced services are growing rapidly due to cost efficiencies and specialized expertise.
- By Region
- North America: Dominates the market owing to the presence of major players, stringent FDA regulations, and high recall rates.
- Europe: Witnessing significant growth due to the implementation of Medical Device Regulation (MDR) and increased compliance demands.
- Asia Pacific: Fastest-growing market driven by manufacturing expansion in China and India, rising device exports, and evolving regulatory frameworks.
- Latin America & Middle East & Africa (LAMEA): Emerging markets with increasing medical device adoption and regulatory modernization.
Competitive Landscape
The medical device complaint management market is moderately consolidated, with leading players focusing on strategic partnerships, product enhancements, and geographic expansion. Key companies include:
- Sparta Systems (a Honeywell Company)
- AssurX
- MasterControl
- IQVIA
- Greenlight Guru
- TrackWise Digital
- Intellect
- Biovia (Dassault Systèmes)
- Veeva Systems
- Pilgrim Quality Solutions
These players offer feature-rich platforms with capabilities ranging from complaint intake, workflow automation, root cause analysis, to integration with electronic health records (EHR) and regulatory reporting systems.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/medical-device-complaint-management-market
Future Outlook
As the global regulatory landscape continues to evolve, complaint management systems will become even more critical. The future of this market will be shaped by:
- Integration with AI and machine learning for smarter decision-making
- Real-time risk assessment based on complaint data trends
- Global harmonization of regulatory reporting standards
- Wider adoption of mobile-enabled and multilingual complaint apps
- Expansion of vendor ecosystems offering end-to-end quality compliance
Manufacturers that invest in modern, scalable, and data-driven complaint management infrastructure will gain a competitive edge in ensuring patient safety, avoiding penalties, and enhancing brand reputation.
Conclusion
The medical device complaint management market sits at the intersection of compliance, technology, and patient care. As regulatory demands intensify and the global medical device landscape grows more complex, robust complaint handling systems will no longer be optional—they will be a business imperative.
Whether it’s managing a product recall, responding to adverse event reports, or streamlining quality investigations, complaint management platforms ensure transparency, traceability, and trust across the device lifecycle. For stakeholders across the ecosystem—from manufacturers to regulators—investing in this domain is not just about compliance; it’s about safeguarding human health and reinforcing the integrity of the medical device industry.
More Trending Latest Reports By Polaris Market Research:
Bottled Water Processing Market
Declutter and Organize Your Garage with the Booming Garage Organization and Storage Market