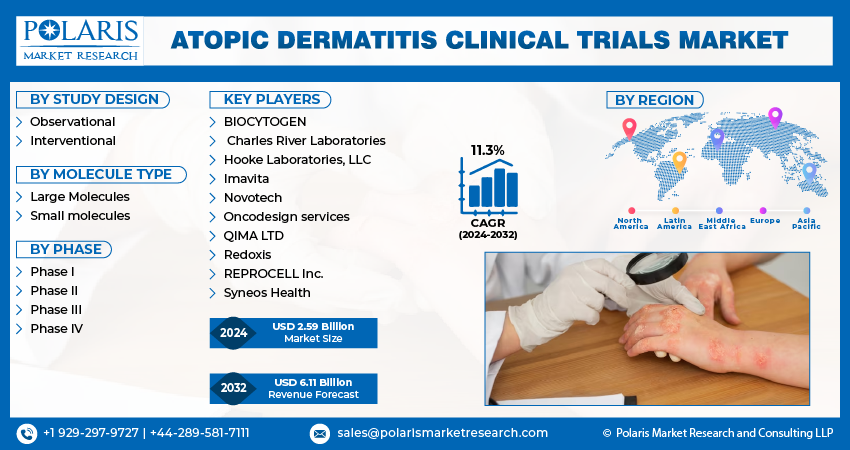
Global Atopic Dermatitis Clinical Trials Market size and share is currently valued at USD 2.59 billion in 2024 and is anticipated to generate an estimated revenue of USD 6.11 billion by 2032, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 11.3% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2024 – 2032.
The atopic dermatitis clinical trials market is witnessing a strong growth trajectory driven by increasing disease prevalence, unmet therapeutic needs, and an expanding pipeline of innovative treatments. Atopic dermatitis, commonly referred to as eczema, is a chronic inflammatory skin condition characterized by itching, redness, and recurring flares. The disease significantly impacts quality of life and poses a long-term burden on patients and healthcare systems alike. As understanding of its complex pathophysiology evolves, pharmaceutical and biotechnology companies are investing in clinical trials for new therapeutic candidates, especially those that target immune system pathways.
The market is undergoing rapid evolution with an increasing number of clinical studies focusing on biologics, small molecules, and novel formulations. From early-phase investigational drugs to large-scale Phase III trials, the global clinical research landscape for atopic dermatitis reflects strong momentum in innovation, regulatory approvals, and patient-centric drug development strategies.
Market Overview
Atopic dermatitis (AD) is one of the most common chronic skin conditions affecting both pediatric and adult populations worldwide. It is characterized by intense itching, dry skin, and inflammation, which may worsen due to environmental or genetic factors. While topical corticosteroids and moisturizers have long been the mainstay of treatment, moderate to severe cases often require systemic therapies or biologics.
In response to rising demand for more effective and safer long-term treatment options, the atopic dermatitis clinical trials market has expanded to include a diverse range of therapeutic approaches. These include monoclonal antibodies, Janus kinase (JAK) inhibitors, PDE4 inhibitors, and immunomodulators, as well as non-pharmaceutical interventions such as phototherapy. Increasing collaboration among academic institutions, contract research organizations (CROs), and biopharmaceutical companies has also accelerated the pace and scale of clinical trials globally.
Key Companies:
- BIOCYTOGEN
- Charles River Laboratories
- Hooke Laboratories, LLC
- Imavita
- Novotech
- Oncodesign services
- QIMA LTD
- Redoxis
- REPROCELL Inc.
- Syneos Health
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/atopic-dermatitis-clinical-trials-market
Polaris Market Research has segmented the Atopic Dermatitis Clinical Trials market report based on study design, molecule type, phase:
Atopic Dermatitis Clinical Trials, Study Design Outlook (Revenue – USD Billion, 2019 – 2032)
- Drug Observational
- Interventional
Atopic Dermatitis Clinical Trials, Molecule Type Outlook (Revenue – USD Billion, 2019 – 2032)
- Large Molecules
- Small molecules
Atopic Dermatitis Clinical Trials, Phase Outlook (Revenue – USD Billion, 2019 – 2032)
- Phase I
- Phase II
- Phase III
- Phase IV
Key Market Growth Drivers
- Rising Global Prevalence of Atopic Dermatitis
The increasing incidence of atopic dermatitis, particularly among children and adolescents, is a major factor driving clinical research in this area. Urbanization, environmental pollutants, and changes in lifestyle and hygiene practices are contributing to the rising disease burden. As a result, there is a growing need for safe, long-term therapies that go beyond symptom management and target the underlying immune dysregulation. - Advancements in Biologic and Targeted Therapies
Recent years have witnessed a paradigm shift in the treatment of atopic dermatitis with the introduction of biologics and other targeted therapies. These agents, which act on specific immune pathways such as IL-4, IL-13, and JAK-STAT signaling, have demonstrated significant efficacy and safety profiles in clinical trials. The success of therapies like dupilumab has opened the door to numerous similar trials, stimulating further investment in the development of next-generation biologics and inhibitors. - High Unmet Need for Moderate to Severe Cases
Despite the availability of topical treatments, a significant proportion of patients with moderate to severe AD experience inadequate disease control and frequent relapses. This treatment gap has created demand for novel therapeutic options that are both effective and well-tolerated over long durations. Pharmaceutical companies are responding by initiating trials to test new mechanisms of action and delivery routes that offer better patient outcomes. - Regulatory Support and Fast-Track Designations
Regulatory agencies in key markets, including the U.S. FDA and the European Medicines Agency (EMA), are supporting the development of AD therapies by offering accelerated pathways, orphan drug designations, and fast-track approvals. These initiatives encourage pharmaceutical companies to invest in clinical trials with the potential to bring breakthrough therapies to market more quickly. This regulatory environment is playing a crucial role in expanding the trial landscape. - Technological Advancements in Clinical Trial Design
The adoption of decentralized and virtual clinical trial models, digital patient monitoring tools, and real-time data analytics has revolutionized the way AD trials are conducted. These innovations improve patient recruitment, reduce trial duration, and enhance data quality, thereby lowering costs and accelerating outcomes. Digital tools also enable greater patient engagement, particularly for pediatric populations, improving compliance and retention. - Strategic Collaborations and CRO Involvement
The growing role of CROs and strategic partnerships among industry players has streamlined clinical trial processes. Collaborations between biotech startups, academic research centers, and major pharmaceutical companies are facilitating multi-center, global studies. This trend is especially significant in expanding access to diverse patient populations and generating more robust clinical evidence for regulatory submissions.
Regional Analysis
North America
North America leads the atopic dermatitis clinical trials market, primarily due to the high prevalence of the condition, strong healthcare infrastructure, and early adoption of novel therapies. The U.S., in particular, is home to numerous ongoing trials evaluating biologics and small molecules. Supportive regulatory frameworks and the presence of leading biopharmaceutical companies make this region a hub for clinical research and innovation.
Europe
Europe holds a significant share in the global atopic dermatitis clinical trials market. Countries such as Germany, the UK, and France are conducting a large number of early- and late-phase trials, often funded by government initiatives or supported through EU-based health projects. Increasing focus on patient-centered care, advancements in dermatological research, and access to public and private research funding are contributing to market growth in this region.
Asia-Pacific
The Asia-Pacific region is emerging as a key market for clinical trials in atopic dermatitis due to its large and diverse patient base, increasing healthcare investments, and cost-effective trial operations. Countries like Japan, South Korea, China, and India are becoming important sites for multinational trials, thanks to growing expertise in clinical research and expanding pharmaceutical infrastructure.
Latin America
Latin America is showing potential for growth in the AD clinical trials market. Brazil, Mexico, and Argentina are leading contributors, with improved regulatory frameworks and access to treatment-naive populations enhancing their attractiveness for global trials. However, challenges such as resource constraints and variable ethics approval timelines may impact trial efficiency.
Middle East and Africa
The Middle East and Africa represent a smaller yet gradually expanding segment of the market. While limited infrastructure and access to dermatological care may hinder growth in certain parts of the region, increasing awareness and investment in healthcare infrastructure in countries like the UAE and South Africa are opening up new opportunities for clinical trial expansion.
Future Outlook
The atopic dermatitis clinical trials market is poised for continued growth as the demand for advanced and effective treatments escalates worldwide. The development pipeline remains robust, with several promising therapies in Phase II and III trials targeting novel biological pathways. Moreover, the continued shift toward personalized medicine and precision dermatology is expected to fuel innovation and broaden the therapeutic landscape.
As clinical trials become more patient-centric, the integration of digital health technologies and artificial intelligence will likely improve trial design, participant engagement, and data analysis. Additionally, the industry is witnessing a trend toward exploring combination therapies and preventative approaches, indicating a maturing market with long-term potential.
In conclusion, the global atopic dermatitis clinical trials market reflects a dynamic environment fueled by scientific breakthroughs, regulatory encouragement, and growing clinical demand. With an increasing number of new entrants and collaborative efforts shaping the research landscape, the market is on track to deliver transformative solutions for millions of patients living with atopic dermatitis.
More Trending Latest Reports By Polaris Market Research:
Waste Recycling Services Market
Pipeline Monitoring System Market