The global market for Isothermal Nucleic Acid Amplification Technology (INAAT) is experiencing rapid growth, driven by increasing demand for fast, accurate, and portable diagnostic solutions. As the healthcare industry pivots toward decentralized testing and early disease detection, INAAT is emerging as a powerful alternative to conventional polymerase chain reaction (PCR) methods, especially in point-of-care settings and resource-limited environments.
Unlike traditional PCR that requires thermal cycling to amplify nucleic acids, INAAT methods amplify DNA or RNA at a constant temperature, enabling faster turnaround times, reduced equipment requirements, and simpler workflows. The most widely used INAAT platforms include loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA), nucleic acid sequence-based amplification (NASBA), and helicase-dependent amplification (HDA). These techniques offer significant advantages in terms of speed, portability, and scalability, positioning them as transformative tools across clinical diagnostics, veterinary testing, food safety, and environmental surveillance.
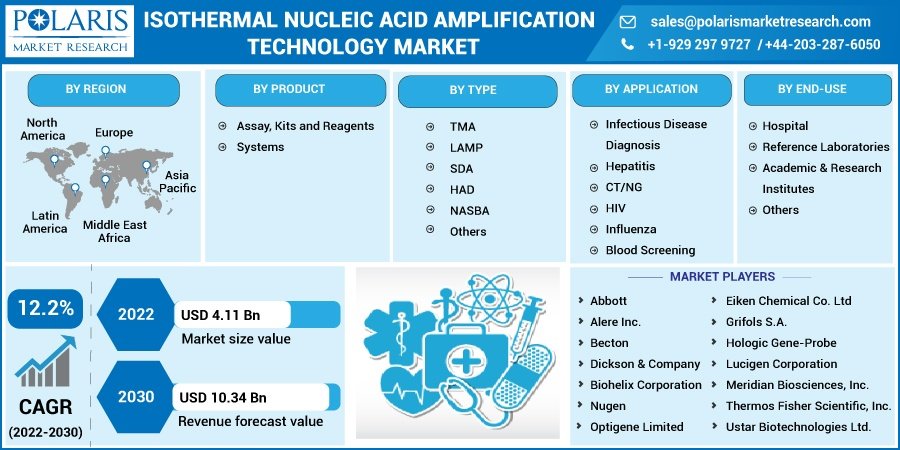
The global isothermal nucleic acid amplification technology market size is expected to reach USD 10.34 billion by 2030, according to a new study by Polaris Market Research. This growth is underpinned by rising applications in infectious disease detection, the continued evolution of molecular diagnostics, and technological advancements that improve assay sensitivity and specificity.
Market Overview
The Isothermal Nucleic Acid Amplification Technology market encompasses a wide range of products and services used for rapid DNA amplification without the need for thermocycling. The core applications span clinical diagnostics, particularly for infectious diseases like COVID-19, HIV, malaria, and tuberculosis, as well as areas like genetic screening, biodefense, and agricultural pathogen detection.
What sets INAAT apart is its ability to deliver results in as little as 15–30 minutes with minimal instrumentation. The simplicity and rapidity of isothermal amplification have led to widespread adoption in field-based diagnostics and low-resource settings where traditional lab infrastructure is lacking. In addition, the compatibility of INAAT platforms with portable and battery-operated devices has made them ideal for point-of-care testing applications in both developed and developing regions.
Market Segmentation
Isothermal Nucleic Acid Amplification Technology, Product Outlook (Revenue – USD Billion, 2018 – 2030)
- Assay, Kits and Reagents
- Systems
Isothermal Nucleic Acid Amplification Technology, Type Outlook (Revenue – USD Billion, 2018 – 2030)
- TMA
- LAMP
- SDA
- HAD
- NASBA
- Others
Isothermal Nucleic Acid Amplification Technology, Application Outlook (Revenue – USD Billion, 2018 – 2030)
- Infectious Disease Diagnosis
- Hepatitis
- CT/NG
- HIV
- Influenza
- Blood Screening
- Others
Isothermal Nucleic Acid Amplification Technology, End-Use Outlook (Revenue – USD Billion, 2018 – 2030)
- Hospital
- Reference Laboratories
- Academic & Research Institutes
- Others
Quick Buy @ https://www.polarismarketresearch.com/buy/1599/2
Regional Analysis
The INAAT market shows a strong presence across major geographic regions, each exhibiting unique adoption drivers and growth patterns.
North America leads the global market, accounting for over 35% of revenue share in 2024. The U.S. has been a pioneer in adopting INAAT for clinical and public health applications, supported by advanced healthcare infrastructure, FDA regulatory support, and high demand for decentralized testing during the COVID-19 pandemic. Ongoing R&D by American biotech firms and academic institutions continues to spur innovation in INAAT platforms.
Europe is another prominent market, with countries like Germany, the UK, and France investing heavily in molecular diagnostics and pandemic preparedness. The region’s strict food safety regulations and growing public health initiatives are driving the adoption of INAAT across healthcare and industrial sectors.
Asia Pacific is emerging as the fastest-growing region, fueled by large-scale infectious disease screening programs, expanding rural healthcare services, and government-backed biotechnology development in China, India, and Southeast Asia. Point-of-care testing is particularly relevant in these areas due to limitations in centralized laboratory infrastructure.
Latin America and the Middle East & Africa are still in the early stages of INAAT adoption but are showing promising growth potential. Public health challenges related to tuberculosis, dengue, and other endemic diseases make INAAT a valuable solution for regional diagnostic needs. International partnerships and NGO-funded programs are playing a pivotal role in introducing isothermal technologies to underserved areas.
Key Companies
The global INAAT market is highly competitive, with leading biotechnology and diagnostic companies actively developing next-generation isothermal platforms. Several players are focusing on making their systems more affordable, user-friendly, and adaptable to a wide range of clinical and field-based settings.
Abbott Laboratories – A key player in INAAT, Abbott’s ID NOW platform has been widely used for COVID-19, influenza, and strep testing. The company continues to expand its INAAT portfolio for various respiratory and sexually transmitted infections.
Eiken Chemical Co., Ltd. – The Japan-based developer of the LAMP technology, Eiken Chemical is a global leader in loop-mediated isothermal amplification systems, with applications in tuberculosis and malaria diagnostics.
Hologic, Inc. – Through its acquisition of Mobidiag, Hologic has entered the INAAT space with platforms like Amplidiag and Novodiag, which combine isothermal amplification with multiplex pathogen detection.
New England Biolabs (NEB) – NEB is a prominent supplier of LAMP and RPA reagents and enzymes, enabling researchers and developers to create customized INAAT workflows.
TwistDx (acquired by Abbott) – Specializes in recombinase polymerase amplification and is instrumental in developing portable diagnostic tests that work at body temperature.
Meridian Bioscience, Inc. – Offers the Revogene platform, which uses isothermal amplification for infectious disease diagnostics in decentralized healthcare settings.
Lucira Health – Known for developing all-in-one, at-home COVID-19 tests using INAAT technology. Lucira’s model demonstrates the potential of INAAT for home-based diagnostics.
𝐆𝐞𝐭 𝐄𝐱𝐜𝐥𝐮𝐬𝐢𝐯𝐞 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐚𝐠𝐞𝐬 𝐨𝐟 𝐓𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭: https://www.polarismarketresearch.com/industry-analysis/isothermal-nucleic-acid-amplification-technology-market/request-for-sample
Future Outlook
The future of the INAAT market looks exceptionally bright, with innovation focusing on miniaturization, multiplexing capabilities, and integration with digital health platforms. Key trends shaping the next phase of growth include:
-
Wearable and portable INAAT devices for rapid field diagnostics in emergency and military applications.
-
AI-driven diagnostics that enhance readout accuracy and reduce human error.
-
Multiplex isothermal assays capable of detecting multiple pathogens in a single test run.
-
Integration with telemedicine platforms for remote diagnosis and treatment initiation.
Additionally, as regulatory bodies worldwide create clearer pathways for point-of-care molecular tests, INAAT platforms will increasingly move from research labs and specialty centers into mainstream clinical workflows and consumer health markets.
Conclusion
Isothermal nucleic acid amplification technology is transforming molecular diagnostics with its speed, simplicity, and adaptability. As healthcare systems continue to prioritize early detection, decentralized care, and pandemic preparedness, INAAT is well-positioned to become the foundation of next-generation diagnostic solutions. With strong commercial momentum, expanding applications, and rapid technological evolution, the INAAT market stands at the forefront of the global movement toward accessible, efficient molecular testing.