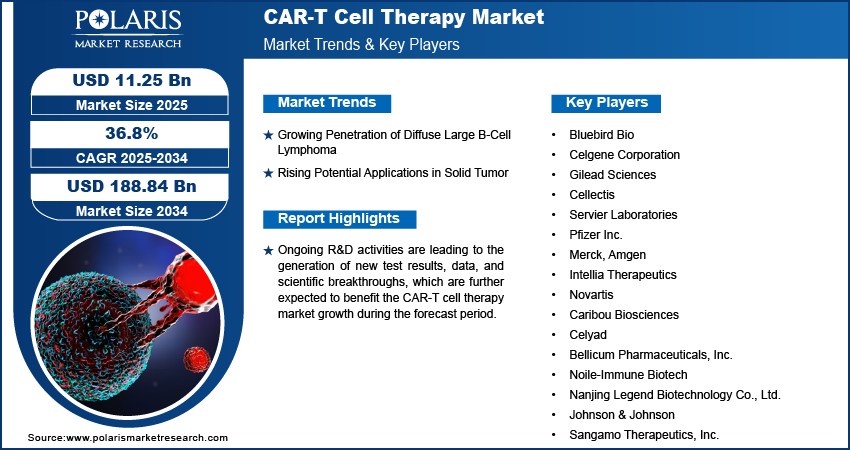
The global CAR-T cell therapy market is experiencing unprecedented growth, projected to expand from USD 11.25 billion in 2025 to approximately USD 188.84 billion by 2034, registering a robust compound annual growth rate (CAGR) of 36.8% during the forecast period.
Market Overview
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents a groundbreaking advancement in immuno-oncology, offering personalized treatment by reprogramming a patient’s T-cells to target and eliminate cancer cells. This innovative approach has demonstrated remarkable efficacy, particularly in hematological malignancies such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). The therapy’s success in achieving complete remission in patients unresponsive to conventional treatments underscores its transformative potential in cancer care.
Key Market Growth Drivers
- Rising Incidence of Hematological Cancers
The increasing prevalence of blood cancers, notably NHL and ALL, has heightened the demand for effective treatments. CAR-T therapy’s targeted approach offers a promising solution, contributing significantly to market expansion.
- Technological Advancements and Product Approvals
Continuous innovations in gene editing and cell manufacturing have streamlined CAR-T therapy production, enhancing its accessibility. The approval of new CAR-T products and expanded indications for existing therapies have further fueled market growth.
- Expansion into Solid Tumors and Autoimmune Diseases
While initially focused on blood cancers, research is extending CAR-T applications to solid tumors and autoimmune conditions. Early clinical trials have shown promising results, indicating a broader therapeutic scope and market potential.
- Strategic Collaborations and Investments
Major pharmaceutical companies are investing heavily in CAR-T research and development, forming strategic partnerships to accelerate innovation and commercialization. These collaborations are pivotal in expanding the therapy’s reach and efficacy.
Market Challenges
- High Treatment Costs
The complex manufacturing process and personalized nature of CAR-T therapy contribute to its high cost, limiting accessibility, especially in low- and middle-income countries.
- Manufacturing and Logistical Hurdles
Producing CAR-T cells involves intricate procedures requiring specialized facilities and expertise. Scaling up production to meet growing demand remains a significant challenge.
- Safety and Side Effects
Patients undergoing CAR-T therapy may experience severe side effects, including cytokine release syndrome and neurotoxicity. Managing these adverse events necessitates careful monitoring and advanced medical support.
- Regulatory and Reimbursement Barriers
Navigating the regulatory landscape and securing reimbursement approvals pose challenges, potentially delaying therapy availability and adoption.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞 @ https://www.polarismarketresearch.com/industry-analysis/car-t-cell-therapy-market
Key Market Players:
- Bluebird Bio
- Celgene Corporation
- Gilead Sciences
- Cellectis
- Servier Laboratories
- Pfizer Inc.
- Merck
- Amgen
- Intellia Therapeutics
- Novartis
- Caribou Biosciences
- Celyad
- Bellicum Pharmaceuticals, Inc.
- Noile-Immune Biotech
- Nanjing Legend Biotechnology Co., Ltd.
- Johnson & Johnson
- Sangamo Therapeutics, Inc.
Polaris Market Research has segmented the CAR-T cell therapy market report on the basis of indication, antigen:
By Indication Outlook (Revenue, USD Billion, 2020–2034)
- DLBCL (Diffuse large B-cell lymphoma)
- ALL (Acute Lymphoblastic Leukemia)
- CLL (Chronic Lymphocytic Leukemia)
- MM (Multiple Myeloma)
- FL (Follicular Lymphoma)
- Mastozytosis
- Myeloid Fibrosis
- MLL (Mixed Lineage Leukemia)
- Thymic Cancer
- Glioblastoma
- AML (Acute Myeloid Leukemia)
- Other
By Target Antigen Outlook (Revenue, USD Billion, 2020–2034)
- CD19/CD22
- BCMA (B-Cell Maturation Antigen)
- Others
Regional Analysis
North America
North America leads the CAR-T cell therapy market, attributed to robust healthcare infrastructure, significant investment in research and development, and favorable regulatory frameworks. The U.S. market, in particular, benefits from early adoption and a high concentration of clinical trials.
Europe
Europe holds a substantial market share, with countries like Germany, the UK, and France at the forefront. The region’s emphasis on advanced healthcare and supportive policies fosters market growth.
Asia-Pacific
The Asia-Pacific region is poised for rapid growth, driven by increasing cancer prevalence, improving healthcare infrastructure, and rising investments in biotechnology. Countries such as China and Japan are emerging as key players in CAR-T research and application.
Latin America and Middle East & Africa
These regions are gradually adopting CAR-T therapies, with growth supported by expanding healthcare access and rising awareness. However, challenges like cost and infrastructure limitations may impede rapid adoption.
Conclusion
The CAR-T cell therapy market is on an accelerated growth trajectory, driven by technological advancements, expanding therapeutic applications, and increasing investment. While challenges persist, ongoing research and strategic collaborations are poised to overcome these hurdles, making CAR-T therapy a cornerstone in the future of personalized cancer treatment.
More Trending Latest Reports By Polaris Market Research:
U.S. Non-Automotive Rubber Transmission Belts Market
Industrial Hemp Market- update
Rapid Oral Fluid Screening Devices Market
Automated Material Handling Equipment Market
mRNA Vaccines and Therapeutics Market
Lawn & Garden Consumables Market
Applicant Tracking System Market