Market Overview:
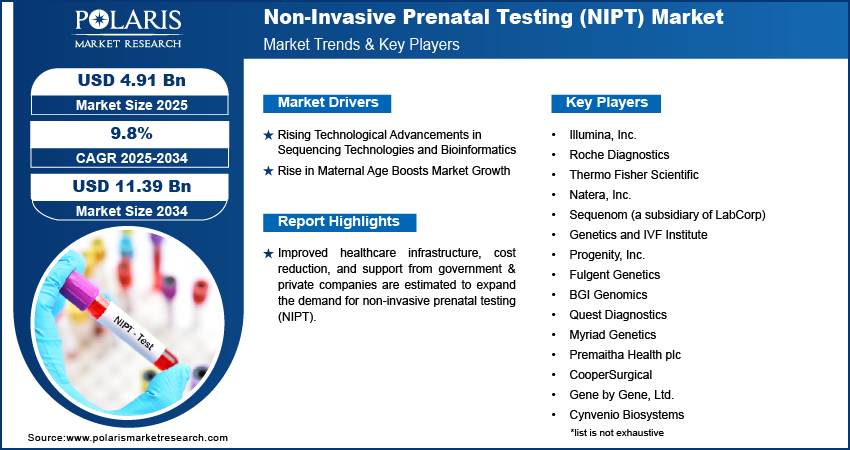
Global Non-Invasive Prenatal Testing (NIPT) Market size and share is currently valued at USD 4.48 billion in 2024 and is anticipated to generate an estimated revenue of USD 11.39 billion by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 9.8% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2025 – 2034
Non-invasive prenatal testing is a revolutionary genetic screening method that analyzes cell-free DNA (cfDNA) fragments in a pregnant woman’s bloodstream to detect the presence of chromosomal abnormalities in the fetus. Unlike amniocentesis or chorionic villus sampling, NIPT poses no risk to the fetus, making it an increasingly popular option for early screening, especially in high-risk pregnancies.
NIPT is typically offered as early as the 9th or 10th week of pregnancy and can provide critical information about the likelihood of conditions such as trisomy 21 (Down syndrome), trisomy 18, trisomy 13, and sex chromosome anomalies. As the technology evolves, its capabilities are expanding to include detection of microdeletions, single-gene disorders, and even whole-genome sequencing applications.
Key Market Growth Drivers:
- Rising Maternal Age and Associated Pregnancy Risks
As more women delay childbirth into their 30s and 40s, the incidence of fetal chromosomal abnormalities has risen. Advanced maternal age is a significant risk factor for genetic anomalies, making NIPT a critical tool for early and accurate detection in this demographic. - Increasing Adoption of Genetic Testing
Growing public and clinical awareness about the benefits of genetic testing during pregnancy is contributing to the wider adoption of NIPT. Healthcare providers are incorporating NIPT into routine prenatal screening protocols, supported by guidelines from medical associations. - Technological Advancements in Genomics
Next-generation sequencing (NGS) and improved bioinformatics platforms have greatly enhanced the sensitivity, speed, and affordability of NIPT. These innovations are enabling laboratories to deliver more comprehensive and accurate reports with shorter turnaround times. - Growing Preference for Non-Invasive Methods
Safety is a paramount concern in prenatal care. The non-invasive nature of NIPT, combined with its high accuracy and early detection potential, has made it a preferred alternative to invasive procedures like amniocentesis, which carry a risk of miscarriage. - Expansion of Healthcare Infrastructure and Reimbursement Policies
Developed countries are increasingly offering insurance coverage or government reimbursement for NIPT, boosting accessibility. Meanwhile, emerging markets are rapidly building capacity for advanced prenatal diagnostics, paving the way for market growth.
Key Companies:
Several companies are leading innovation and commercialization in the NIPT space through technological advancements, strategic partnerships, and global expansion. Prominent players include:
- Agilent Technologies, Inc.
- Centogene N.V.
- Eurofins LifeCodexx GmbH
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Laboratory Corp. of America Holdings
- MedGenome Labs Ltd.
- Myriad Women’s Health, Inc.
- Natera, Inc.
- Progenity, Inc.
- Qiagen
- Quest Diagnostics, Inc.
- Thermo Fisher Scientific, Inc.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐓𝐡𝐞 𝐂𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐂𝐨𝐦𝐩𝐫𝐞𝐡𝐞𝐧𝐬𝐢𝐯𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐇𝐞𝐫𝐞:
https://www.polarismarketresearch.com/industry-analysis/non-invasive-prenatal-testing-nipt-market
Market Segmentation:
By Gestation Period Outlook (Revenue – USD Billion, 2020–2034)
- 0-12 weeks
- 13-24 weeks
- 25-36 weeks
By Component Outlook (Revenue – USD Billion, 2020–2034)
- Consumables
- Instruments
- Software
- Services
By Method Outlook (Revenue – USD Billion, 2020–2034)
- Cell-Free DNA in Maternal Plasma Tests
- Ultrasound Detection
- Biochemical Screening Tests
- Others
By Application Outlook (Revenue – USD Billion, 2020–2034)
- Monosomy
- Trisomy
- Microdeletion Syndrome
- Others
By End Use Outlook (Revenue – USD Billion, 2020–2034)
- Diagnostic Centers
- Hospitals and Clinics
- Others
Regional Analysis:
North America holds the largest share of the NIPT market, led by the United States. The region benefits from well-established healthcare infrastructure, favorable reimbursement policies, high maternal age averages, and the presence of major market players. The adoption of advanced genetic testing technologies in routine care has also contributed to market dominance.
Europe follows closely, with strong demand in countries such as Germany, the UK, and France. Supportive regulations and clinical guidelines that promote early screening, combined with public health campaigns, are fostering steady growth.
Asia-Pacific is poised to be the fastest-growing regional market, driven by a large population base, increasing urbanization, rising awareness of prenatal care, and government efforts to modernize healthcare systems. Countries like China, India, and Japan are investing in genomics research and expanding their diagnostic capabilities.
Latin America and the Middle East & Africa are emerging markets with growing demand due to rising maternal health awareness and increasing availability of advanced diagnostic services. While infrastructural and economic barriers persist, international collaborations and public-private partnerships are helping bridge the gap.
Future Outlook:
The future of the Non-Invasive Prenatal Testing (Nipt) Market is bright, as technological advancements continue to make the tests more comprehensive, accessible, and affordable. Ongoing research into single-gene disorder detection, integration with artificial intelligence, and genetic testing for rare conditions will open new frontiers in prenatal care.
As consumer interest in personalized medicine grows, and healthcare providers increasingly prioritize preventive screening, NIPT will play a central role in ensuring healthier pregnancies and early intervention strategies. The continued expansion of testing into low- and middle-income countries will further accelerate the global adoption of cell-free DNA screening as a standard component of prenatal diagnostics.
More Trending Report:
Durable Medical Equipment Market
Artificial Intelligence in Precision Medicine Market
Organoids and Spheroids Market